When talking about galvanized vs zinc-plated steel, most often people are actually referring to hot-dipped galvanized steel and zinc-plated steel.
Galvanized steel is a generic term for steel with a zinc coating. This zinc coating helps protect the metal from the elements and the moisture in the air.
Two popular galvanization methods include zinc-plating and hot-dip galvanization.
These methods can be used on bolts, screws, nails, washers, nuts, chains, pipe, and more.
What is Zinc-Plated Steel?
Zinc plated steel is plain steel that is coated with a very thin layer of zinc by using electricity. It’s main purpose is to prevent the steel from rust and corrosion.
Zinc plated steel is easy to spot as it is usually shiny silver and slightly blue in color. Although, yellow zinc and black zinc plating also exist and can offer better corrosion resistance than “blue zinc”.
If you walk through the fastener isle at your local hardware store you will see a large amount will be zinc plated.
Typical zinc plating will take plain steel that looks like this:

And turn it into this:

How is Zinc-Plated Steel Actually Plated?
The zinc plating process varies slightly from manufacturer to manufacturer. However, the process described below is one used by Surface Treatment Experts and is typical of many manufacturers.
Cleaning
Materials to be zinc plated, aka substrate, are first thoroughly cleaned of any debris, corrosion, or oils.
Plating Solution
A plating solution, or bath, is then mixed up for the substrate to be immersed in. This bath is basically an electrolyte solution mixed with zinc ions (charged zinc atoms). Often there are other chemicals included as well. These chemicals adjust the speed and distribution of the zinc onto the substrate.
Electrodeposition
A DC current is then applied to the solution. The current flows from the anode to the substrate, which serves as the cathode. During this process, the zinc ions are deposited onto the substrate. The current then flows back to the anode and the cycle repeats:
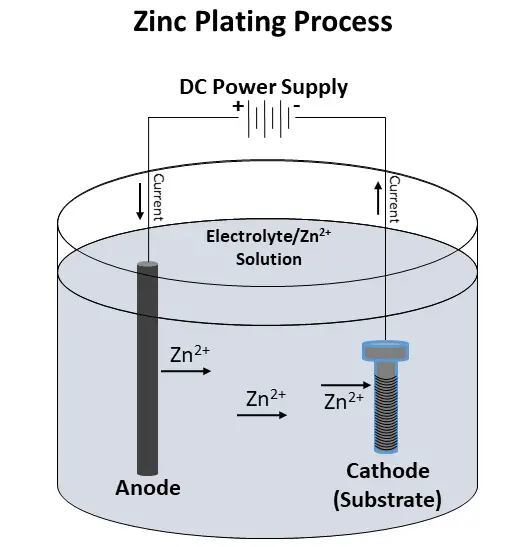
Post Treatment
Finally, the substrate is removed from the solution, rinsed, and dried. Passivation is a process often used to protect the zinc. This can also alter the final color. Sometimes, the substrate is sealed with a clear sealer as well to add extra corrosion protection.
Zinc Plating Thickness
The thickness of zinc plating ranges from 5 to 25 µm. One µm (micrometer) is 0.000001 meters.
The actual thickness depends on the standard to which is manufactured to. Listed below are four classes of zinc-plating, defined by ASTM B633:
-
- Fe/Zn 5 (5 µm thickness)
- Fe/Zn 8 (8 µm thickness)
- Fe/Zn 12 (12 µm thickness)
- Fe/Zn 25 (25 µm thickness)
Hot-Dip Galvanized vs Zinc-Plated Steel
Hot-dip galvanized steel is also coated with zinc, but a different manufacturing process is used.
During the hot-dip galvanization process, metal parts are actually submerged in molten zinc. This provides a thicker and longer lasting coating than electrodeposition.
This process is often used on fasteners, hardware, and building materials that will be exposed to the elements and used outdoors. Things like wood fences, for example, are almost always built using hot-dip galvanized fasteners. On the other hand, zinc-plating is the preferred coating for something like framing a wall.
Hot-dip galvanized parts have a dull, flat grey appearance. Hot-dip galvanized steel looks like this:

Zinc Nickel Plating
Zinc nickel plating is a superior variation of zinc plating. Here, nickel gets combined with zinc in the electrodeposition process.
Although nickel increases the corrosion resistance, it is a more expensive process and therefore not as widely used.
Galvanized and Zinc-Plated Steel Restrictions
High Temperatures
Don’t use zinc coated materials in applications with temperatures of 500°F and higher. This includes welding. The zinc coating will create toxic fumes that can lead to serious health problems. Always make sure to remove any zinc coatings prior to welding.
Acidic and Basic Environments
Zinc coatings do not hold up well against acids or alkalis. Use different coatings, like powder or plastic coating, in these environments.
Marine Environments
The salty environment in many marine applications can inhibit zinc’s durability as well. Use other coatings in these environments.
